Background: Numerous reports indicated the significance of the PI3K/AKT/mTOR signaling pathway in AML. Up to now, there is no FDA-approved PI3K/AKT/mTOR inhibitors available for patients with acute myeloid leukemia (AML). Recently, we demonstrated that PI3K/mTOR dual inhibitor omipalisib had a potent anti-leukemic effect, but it could not completely eliminate leukemia cells in a subcutaneous murine model. To explore the refractory features of omipalisib, we established several OCI-AML3 sublines refractory to omipalisib by an in vivo selection procedure. The OCI-AML3-R cell line was the most refractory one and showed cross-resistance to another PI3K/mTOR inhibitor dactolisib and multi-tyrosine kinase inhibitor dasatinib, in addition to omipalisib. Several genes associated with multidrug-resistance, ABCC1, ABCC2, ABCB1, ABCG2, MDR1, and MRP1, did not express significant difference between OCI-AML3 and OCI-AML3-R.
Aims: To investigate the molecular mechanisms of OCI-AML3-R using transcriptomics and metabolomics analysis, and to explore potential therapeutic strategies from both in vitro and in vivo aspects.
Methods: OCI-AML3 and OCI-AML3-R leukemia cell lines were used in this study. MTS assay was performed to assess cell viability and proliferation. The differential expression genes (DEGs) were examined using RNA-seq (Illumina NovaSeq 6000). Metabolomic analysis was conducted by the C-SCOPE service using capillary electrophoresis mass spectrometry CE-TOFMS and CE-QqQMS with Agilent 6460 TripleQuad LC/MS (HMT, Yamagata, Japan). Q-RT-PCR was used for mRNA quantification. Immunoblotting were used to clarify the variations of total- and phosphor-proteins. Flow cytometry was used for apoptosis and mitochondrial ROS content analysis. OCI-AML3-R xenograft models in nude mice were used for scrutinizing efficacy of PHGDH inhibitor in vivo. The protocol for the xenograft experiments was approved by the IACUC, National Taiwan University.
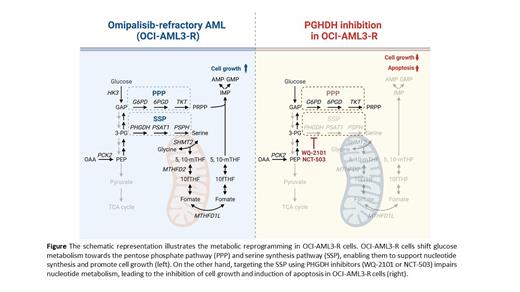
Results: A total of 1946 DEGs between OCI-AML3 and OCI-AML3-R cells were identified with the selection criteria of p< 0.05 and [log 2 (fold change)]> 1 or <1, of which 477 and 1469 DEGs were up- and down-regulated, respectively. g:Profiler was used for functional enrichment analysis and revealed that NFkB signaling-related pathways ( p< 0.001), cell cycle-related pathways ( p< 0.001), and nucleotide metabolic pathways ( p< 0.001) were enriched in OCI-AML3-R. Q-RT-PCR confirmed that a series of genes related to pentose phosphate pathway (PPP; TKT and 6PGD), serine synthesis pathway (SSP; PHGDH and PSAT1), purine synthesis and folate/methionine cycle ( PAICS, SHMT2, MTHFD2, and MTHFD1L) were increased in OCI-AML3-R cells. Among them, PHGDH and PSAT1 increased the most, 4.75x and 11.38x, respectively. A total of 103 metabolites were screened out by metabolomic analysis, 58 of which showed significant differences between OCI-AML3 and OCI-AML3-R cells ( p< 0.05). Metabolite enrichment analysis also revealed that the purine metabolism, urea cycle, glycolysis/gluconeogenesis, and several amino acid metabolism pathways were enriched in OCI-AML3-R cells. A comprehensive overview of metabolites in glycolysis and TCA cycle in OCI-AML3-R cells revealed significantly increased levels of PRPP and serine, as well as steady-state levels of glycine, folate, and several purine metabolites. These results indicated that a reprogramming of glucose metabolism towards the PPP and SSP in OCI-AML3-R cells was noted. Based on these results, we proposed targeting the PPP and SSP as a therapeutic approach for omipalisib-refractory AML. The PHGDH inhibitors, WQ-2101 and NCT-503, showed strong anti-leukemic effects in OCI-AML3-R with an IC 50 of 26.17 and 16.81 μM, respectively, while the G6PD inhibitor 6-AN had limited effect. The PHGDH inhibitors increased mitochondrial ROS levels and induced apoptosis in OCI-AML3-R. Additionally, in vivo experiment showed that treatment with NCT-503 significantly prolonged mouse survival and inhibited tumor growth in a dose-dependent manner without adverse effects on body weight.
Summary/Conclusion: Transcriptomic and metabolomic analyses revealed that OCI-AML3-R upregulated PPP and SSP, leading to increased nucleotide synthesis, which contribute cell growth and survival. Targeting PHGDH could significantly suppress omipalisib-refractory in AML and may have implications for future clinical trials.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal